Do you desperately look for 'how to write valence orbital notation'? You can find your answers here.
Table of contents
- How to write valence orbital notation in 2021
- Electron orbital diagram
- How to write orbital notation
- What are valence orbitals
- Valence electron orbital diagram
- Orbital valence shell
- Valence orbital diagram
- Orbital diagram calculator
How to write valence orbital notation in 2021
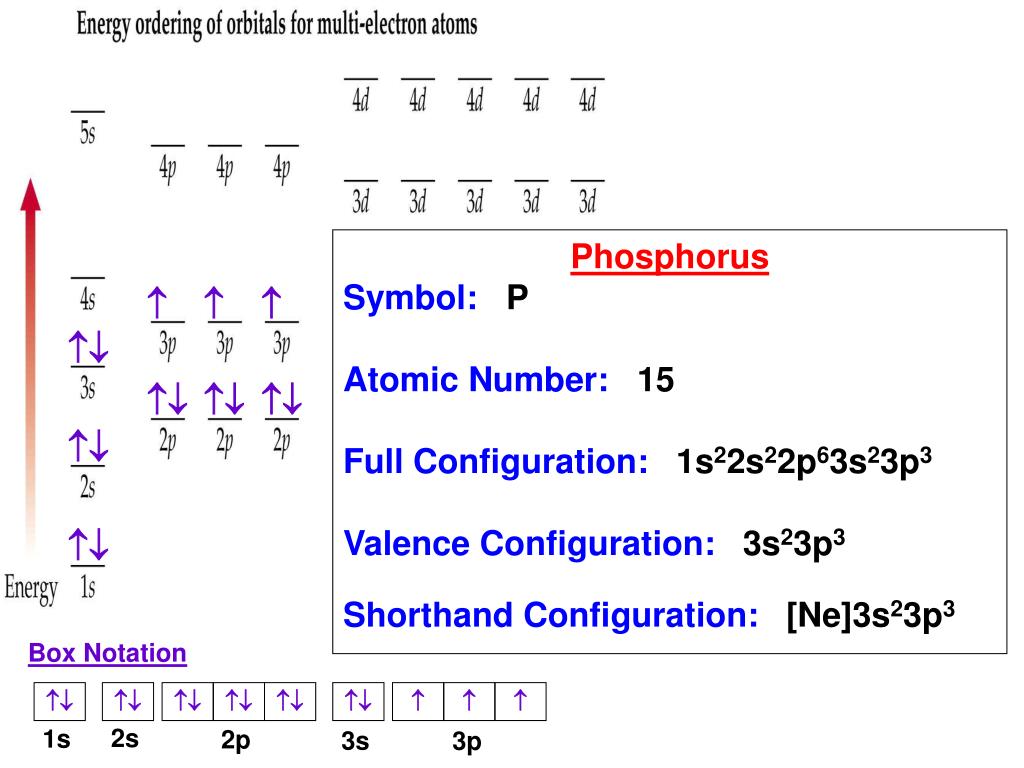 This image demonstrates how to write valence orbital notation.
This image demonstrates how to write valence orbital notation.
Electron orbital diagram
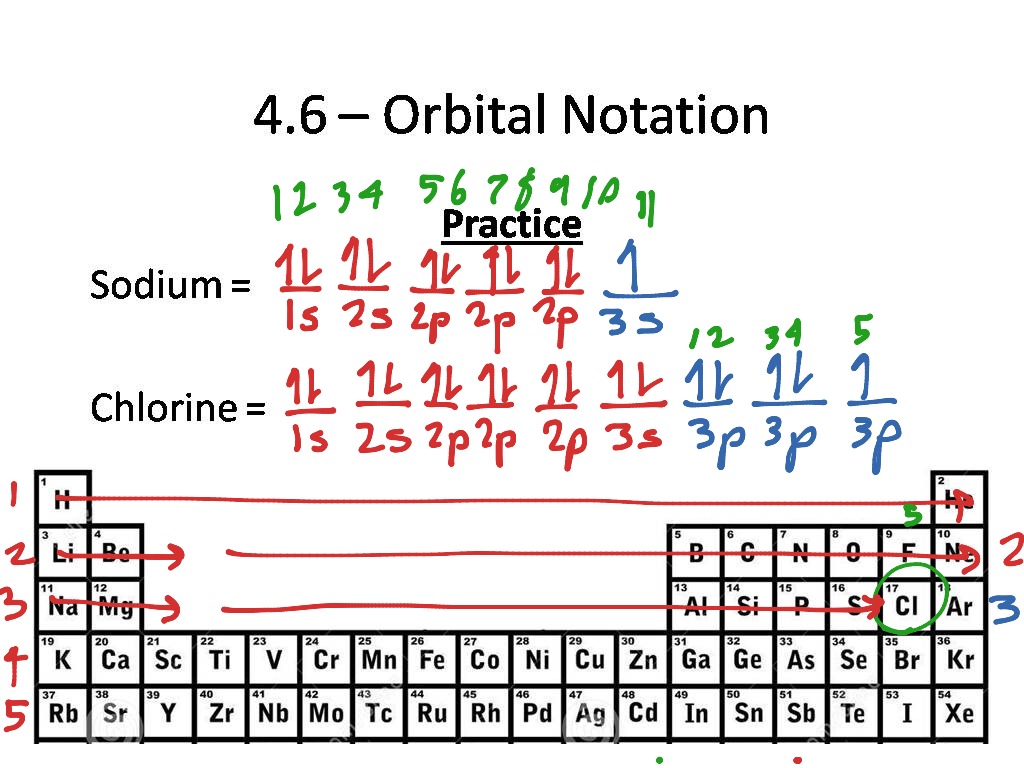 This image shows Electron orbital diagram.
This image shows Electron orbital diagram.
How to write orbital notation
 This picture demonstrates How to write orbital notation.
This picture demonstrates How to write orbital notation.
What are valence orbitals
 This picture shows What are valence orbitals.
This picture shows What are valence orbitals.
Valence electron orbital diagram
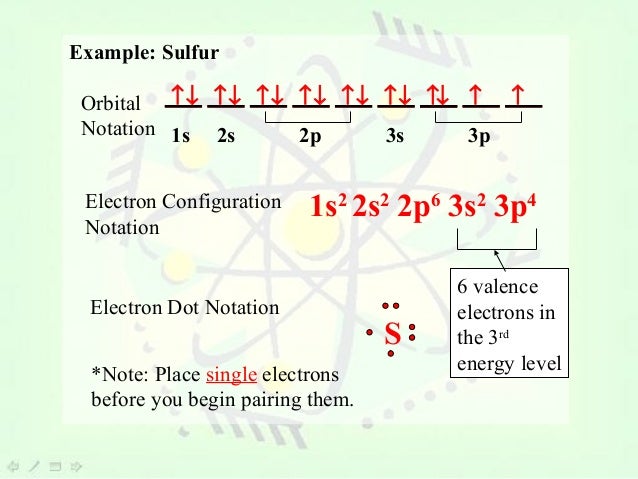 This picture shows Valence electron orbital diagram.
This picture shows Valence electron orbital diagram.
Orbital valence shell
 This image demonstrates Orbital valence shell.
This image demonstrates Orbital valence shell.
Valence orbital diagram
 This picture shows Valence orbital diagram.
This picture shows Valence orbital diagram.
Orbital diagram calculator
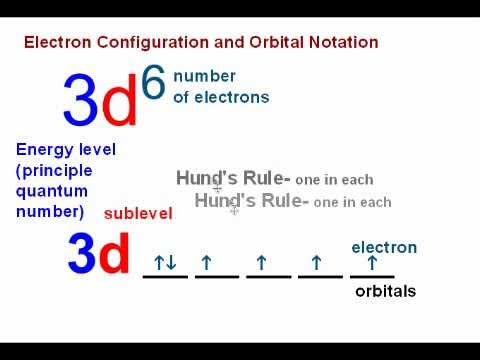 This picture representes Orbital diagram calculator.
This picture representes Orbital diagram calculator.
How to calculate the number of electrons in an orbital?
TL;DR (Too Long; Didn't Read) Electron configurations have the format: 1s 2 2s 2 2p 6 . The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital.
How are the valence electrons of an element determined?
Each element has a number of valence electrons equal to its group number on the Periodic Table. This table illustrates a number of interesting, and complicating, features of electron configuration. First, as electrons become higher in energy, a shift takes place.
What's the best way to do an orbital diagram?
How to Do Orbital Diagrams 1 Electron Configurations. Electron configurations are expressed through a notation that looks like this: 1s 2 2s 2 2p 1. ... 2 Shorthand Notation for Configuration. Writing out every single orbital for heavier elements is tedious, so physicists often use a shorthand notation. 3 Orbital Diagrams. ... 4 Dot Diagrams. ...
Is there a way to write orbital notation?
(Playback ID: F8TxNi_lI7t2w1Ly) An error occurred while retrieving sharing information. Please try again later.
Last Update: Oct 2021